To most of us, the concept of a battery is nothing new—we know batteries are the way to store electrical (DC) energy for future use.
But while the concept is easy to comprehend, understanding how a battery works can be less clear.
More importantly, battery designs can vary greatly from application to application. (Hence why the battery in your smartphone is different than the battery under your hood.)
That’s why we liked this video from the folks over at Yuasa. They’ve been making lead-acid batteries for motorcycles, cars, boats, and trucks for decades. So it’s a good place to start when asking about battery construction.
The video takes a 101-style approach to battery design and walks you through a basic overview of how a lead-acid battery works.
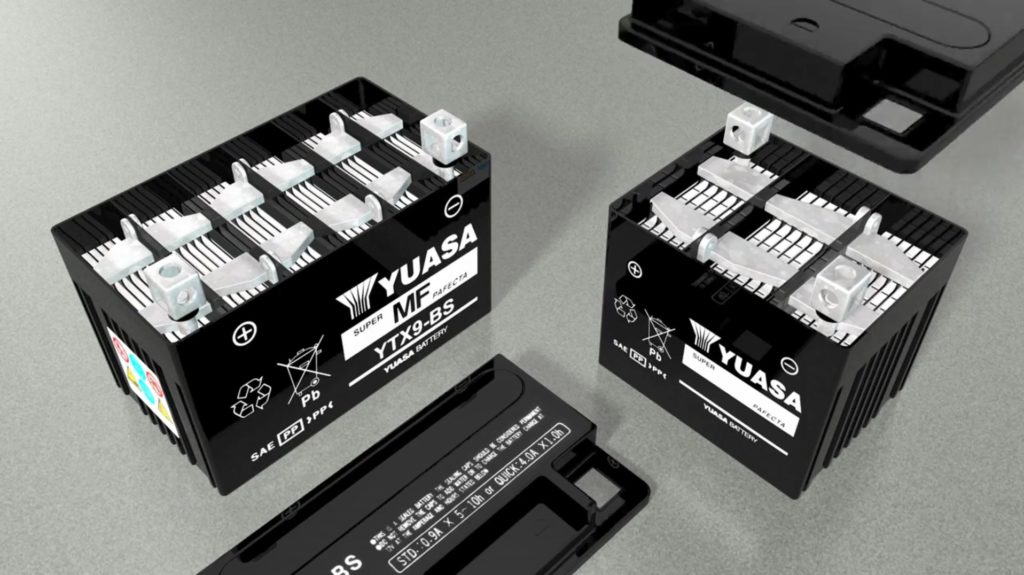
It includes some exploded views of a battery’s internals, so you can see how the components relate, and perhaps give you a better idea on how to troubleshoot a dead battery.
After you’ve watched the video, the series continues with a nice tutorial on how lead-acid batteries work.

What causes an otherwise strong battery to develop a dead cell? What can I do to prevent this?
Well Fred, a dead cell can have several causes. Here are a few: damage from an impact (like dropping it on a shop floor), the loss of electrolyte (from heat or lack of maintenance), or contamination inside the battery.
How can you prevent it? We’ve got two posts on battery maintenance, you can check them out here and again here.